Embedded Cybersecurity
Ensuring Medical Device Security
Velentium's cybersecurity team covers all aspects of medical device product security - we are here to help you rapidly bring a secure product to market - and monitor it afterwards
We can provide every activity and document as required by the September 2023 FDA cybersecurity guidance for device submission
Security Plan
Commitment to both pre and post market cybersecurity activities.
Risk Management
Threat modeling of assets and processes. Security architecture views.
Supply Chain
Human and machine readable Software Bill Of Materials (SBOM). SBOM support report. Vendor assessments.
Online Training
A self paced video training with certification for medical device developers (for more details and sign up here).
Security Controls
Authentication, authorization, cryptography, integrity, confidentiality, event detection, resiliency, & update.
Testing
Fuzz testing, malformed inputs, attack surface analysis, penetration testing, vulnerability scanning, software content analysis, static analysis.
Labeling
Content to be added to the Instructions For Use (IFU) to address all 14 areas of transparency to the end user as required by the FDA.
Governance
Drafting corporate policy and procedures to comply with FDA, US Federal Government, and other international requirements (including EU's MDR).
Postmarket Activities
Fulfillment and periodic security upgrades to off the shelf (OTS) components
Ongoing surveillance: subscription service to assure your device remains secure and compliant with FDA postmarket requirements.
This service includes (as required by FDA): Periodic reperformance of cybersecurity tests & SBOM monitoring
Medical device incident response support team: assisting you in the event of adverse situations
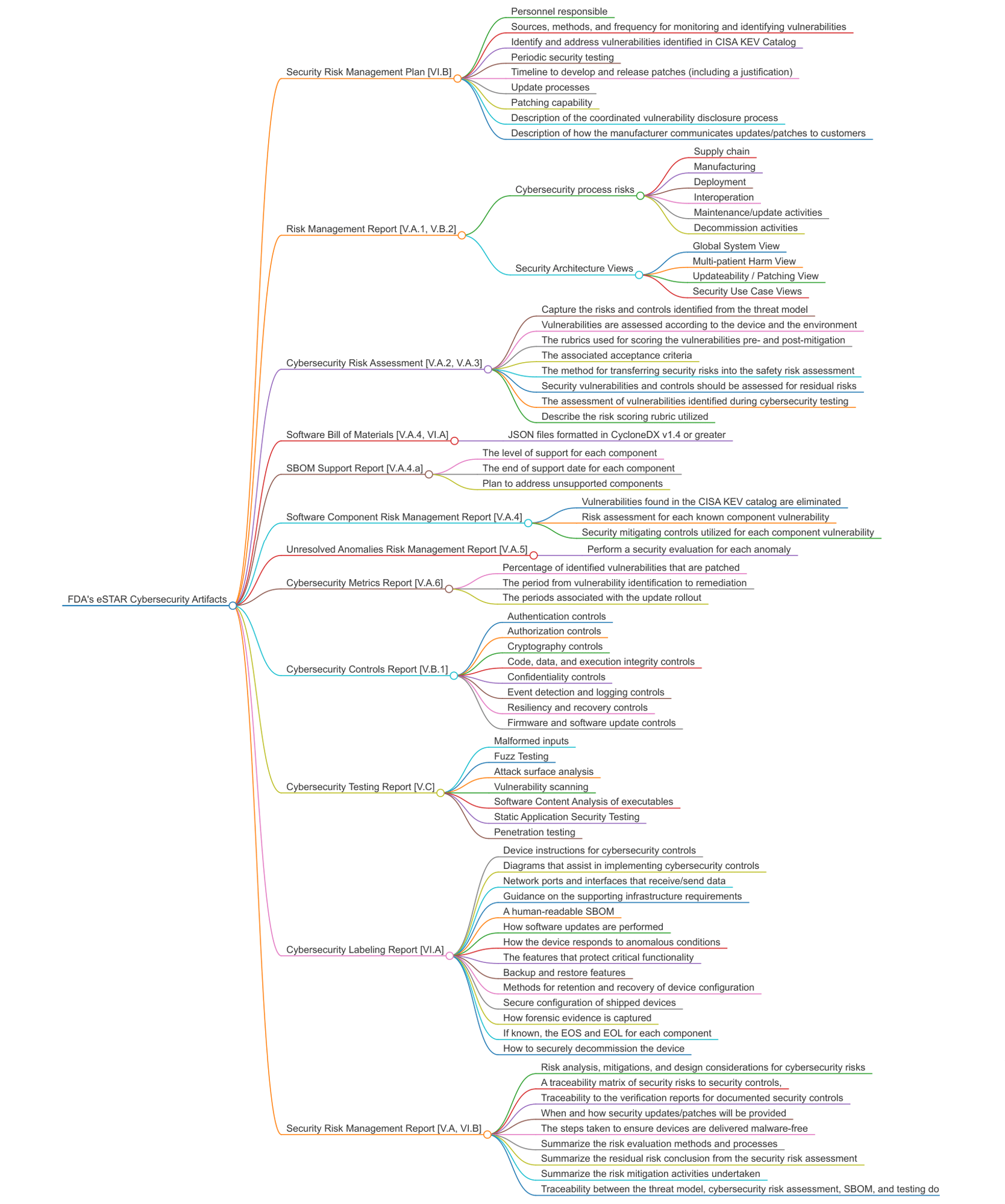
Every medical device's premarket submission (if it has a software component) must supply the complete list of FDA required artifacts as shown here.
Our assistance can range from guiding your team to handling it all on your behalf.
Meet Our Experts
Network security is different than embedded product security. And iOT product security is different than medical product security. Our team has decades of medical device embedded product development and cybersecurity experience.

Safe. Secure. Effective.
One stop for secure Medical Device R&D, product development, contract manufacturing, and postmarket services





